Understanding ELISA Assay
Enzyme Linked Immunosorbent Assay or ELISA assay is a method widely utilized for quantitation and identification of protein, peptides, hormones, and antibodies. In ELISA assay formation, an antigen and antibody are complexed together, where the antigen is immobilized or attached to a solid surface and the antibody is associated with the enzyme. The detection phase of the ELISA assay is accomplished by incubating the enzyme with a substrate and then evaluating the conjugated enzyme reaction. During detection, antibody-antigen contact or reaction is an essential element.
The process of Enzyme linking was developed in the 1960s by two teams, headed by G.B Pierce and Stratis Avrameas respectively. Chromogenic reporters and substrates are used in conventional ELISA assays to produce color changes that would indicate the presence of a specific analyte or antigen. Electrochemiluminescent, fluorogenic, and quantitative PCR reporters are used in newer Assay methodologies to create signals that are quantifiable and measurable.
The basic principle of ELISA is the coupling of antigens or antibodies to the assay enzyme. The specificity of an antibody is combined with the sensitivity of an assay enzyme in order to perform one primary function – detect antibodies through assay antigens or antigens through assay antibodies. The precision and sensitivity of the assay can be further enhanced by using high-affinity antibodies as a coating layer for the plate.
ELISA is usually performed on a polystyrene plate with 96-well or 384-well, which binds proteins and antibodies. The immobilization and binding together make ELISA simple to perform and easy to create. When ELISA reactants are immobilized on the surface of microplates, it becomes easier to separate non-bound and bound materials from each other. The materials that are nonspecifically bound are washed away from the plate, which allows the measurement of specific analytes.
ELISA Principle
The principle of ELISA assay is similar to other immunoassay technologies. It relies on the method in which antigen and antibodies bind together and an efficient detection system is utilized to identify the quantity and presence of antigen binding.
The plate used in the ELISA assay is carefully coated using antibodies of high-affinity so that the precision and sensitivity of the assay can be maximized.
ELISA Assay Formats
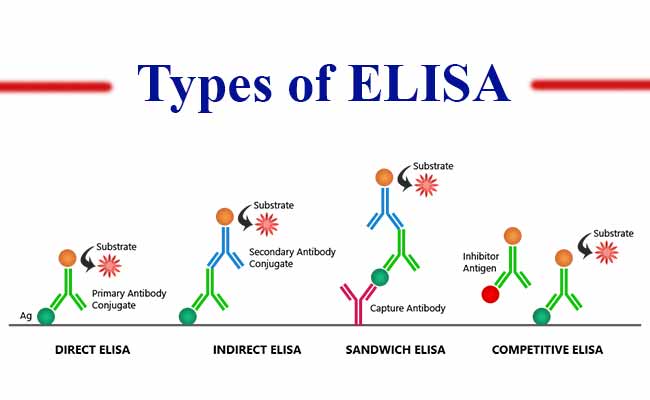
ELISA assay is often performed with a few modifications such as indirect or direct assay plate absorption. Below we have explained some of the common types of ELISA assay formats:
1. Direct ELISA Assay
During direct ELISA detection, antibodies and enzymes are directly conjugated and this is utilized to detect the antigen which is coated on the specific assay plate.
Advantages
2. Indirect ELISA Assay
During indirect ELISA assay, antigens, which are coated on the ELISA plate, follow two-stage detection. First, a primary antibody which is not labeled is applied. This antibody is explicit for the antigen. Secondly, the primary antibody is bound with a secondary antibody that is labeled with an enzyme. This secondary antibody is usually polyclonal and anti-species.
Advantages
3. Sandwich ELISA
In sandwich ELISA assay, antibody pairs are utilized, where both the antibodies are specific to the epitope or non-overlapping portion of the antigen. The first antibody, which is commonly referred to as the capture antibody, is glazed along with the wells. Then, the well is filled with a sample solution. The second antibody, which is commonly referred to as the detection antibody, is used to measure the sample concentration.
Advantages
Data Interpretation in ELISA Assay
The ELISA assay leads to three data outputs, which are explained below:
Conclusion
In the biopharma or pharmaceutical industries, ELISA assays are commonly utilized to detect antigens in the blood. It helps in detecting allergens, hormones, viral antigens, antibodies, and bacterial antigens produced by the body in response to an infection. This method is also utilized for drug discovery and development, its preclinical and clinical phases, to characterize proteins or to form new drug therapies.